Estimated reading time: 20 minutes
Let us explore the patient guide to US FDA-approved CAR T therapies, dosage, safety concerns and risk management (REMS), efficacy in clinical trials, and impact.
CAR T cell therapy is approved by the US FDA to treat defined conditions of leukemia, lymphoma, and multiple myeloma. It is often used as a last resort, after one or more prior lines of therapy. However, CAR T cell therapy has had remarkable success measured by overall response rates in patients.
Table of contents
- CAR-T therapy: What are T cells
- What is CAR
- The science behind CAR T therapy
- US FDA approved CAR T therapies
- ABECMA CAR T therapy for multiple myeloma
- What exactly does ABECMA do?
- Multiple myeloma clinical indications for ABECMA CAR-T therapy
- Dosage and administration of ABECMA CAR T therapy in multiple myeloma
- Safety concerns and risk management of ABECMA CAR-T therapy in multiple myeloma
- Efficacy of ABECMA CAR T Cell therapy in multiple myeloma
- Impact of ABECMA therapy in multiple myeloma
- BREYANZI CAR T therapy for Lymphoma
- Defining BREYANZI: A Precision Approach
- Lymphoma clinical conditions for BREYANZI CAR-T therapy
- Dosage and Administration of BREYANZI CAR T therapy in lymphoma patients
- Safety concerns and risk management of BREYANZI CAR T therapy in lymphoma patients
- Efficacy of BREYANZI CAR T cell therapy in lymphoma
- Impact of BREYANZI therapy in lymphoma
- CARVYKTI CAR T therapy for multiple myeloma
- Multiple myeloma clinical indications for CARVYKTI CAR-T therapy
- Dosage and administration of CARVYKTI CAR T therapy in multiple myeloma patients
- Safety concerns and risk management of CARVYKTI CAR T therapy in multiple myeloma patients
- Efficacy of CARVYKTI CAR T cell therapy in multiple myeloma
- Impact of CARVYKTI therapy in multiple myeloma
- KYMRIAH CAR T therapy for lymphoma
- What is KYMRIAH Therapy?
- Lymphoma clinical indications for KYMRIAH CAR-T therapy
- Dosage and administration of KYMRIAH CAR T therapy in lymphoma patients
- Safety concerns and risk management of KYMRIAH CAR T therapy in lymphoma patients
- The efficacy of KYMRIAH CAR T cell therapy in lymphoma
- Impact of KYMRIAH therapy in lymphoma
- TECARTUS CAR T therapy for lymphoma and leukemia
- Understanding TECARTUS CAR-T Therapy
- Lymphoma and leukemia clinical indications for TECARTUS CAR-T Therapy
- Dosage and Administration of TECARTUS CAR T Therapy in lymphoma and leukemia patients
- Safety concerns and risk management of TECARTUS CAR T Therapy in lymphoma and leukemia patients
- Efficacy of TECARTUS CAR T cell therapy in lymphoma and leukemia patients
- Impact of TECARTUS Therapy in lymphoma and leukemia patients
- YESCARTA CAR T therapy for lymphoma
- What is YESCARTA Therapy?
- Lymphoma clinical indications for YESCARTA CAR-T therapy
- Dosage and Administration of YESCARTA CAR T therapy in lymphoma patients
- Ensuring Safety and Management of YESCARTA CAR T therapy in lymphoma patients
- Efficacy of YESCARTA CAR T cell therapy in lymphoma patients
- Impact of YESCARTA in lymphoma patients
- Ongoing CAR-T cell therapy in Clinical trials
- The conditions currently being evaluated
- Managing adverse and side effects
- Conclusion
- FAQ
CAR-T therapy: What are T cells
It is combination of cell and gene therapy – T Cells and CAR.
At the core of CAR T therapy lies the perfect fusion of cell and gene therapy —T Cells and CAR. T cells are often referred to as the immune system’s defenders. T cells are adept at identifying and removing foreign invaders like viruses, bacteria, and cancer cells.
Cytotoxic T cells among the T cell population serve as vigilant warriors, relentlessly seeking out and destroying cancerous or tumor cells.
What is CAR
Chimeric Antigen Receptor (CAR) is a marvel of modern science. CAR is an engineered synthetic receptor to target specific receptors present in cancer cells.
CAR equips T cells with a newfound ability to recognize and lock onto cancer cells, triggering a potent anticancer response.
The science behind CAR T therapy
In essence, CAR T therapy is often referred to as “B-cell maturation antigen (BCMA) or CD19-directed genetically modified autologous T cell immunotherapy
CAR T therapy involves apheresis, where medical professionals collect a tissue sample from the patient and isolate the specialized T cells. Further, T cells are genetically engineered to express CAR on their surface. Subsequently, CAR T cells multiplied into millions within the confines of a laboratory. Finally, the engineered CAR T cells were administered to the patient.
Next, the CAR T cells embark on a targeted quest, explicitly seeking out cancer cells expressing CD19 and/or B-cell maturation antigen (BCMA). Further, the interaction between CAR T and cancer cells initiates a cascade of events, leading to an impressive display of anticancer activity.
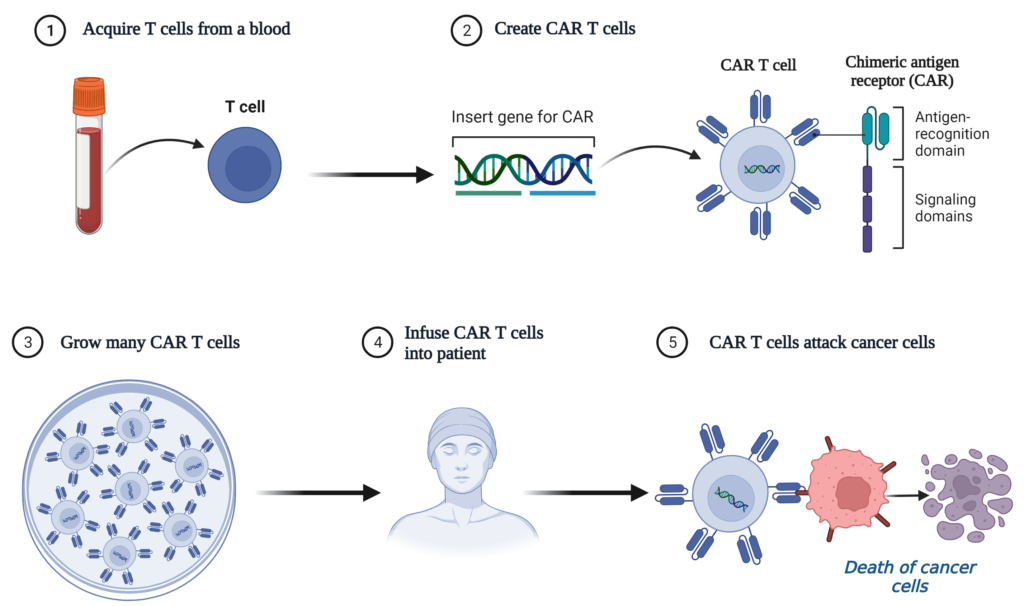
So, join us as we delve into the world of cutting-edge therapies, exploring the inspiring potential of US FDA-approved CAR T therapies in revolutionizing cancer care.
US FDA approved CAR T therapies
ABECMA CAR T therapy for multiple myeloma
In medical advancements, breakthrough therapies have emerged to provide hope and new possibilities for patients battling serious diseases. One such remarkable treatment is ABECMA, a groundbreaking autologous cell therapy that utilizes the power of immunotherapy.
ABECMA falls under the category of autologous treatment. You might have also heard it referred to as CAR T therapy.
| Name | Trade name | Manufacturer |
| idecabtagene vicleucel | ABECMA | Celgene Corporation, a Bristol-Myers Squibb Company |
What exactly does ABECMA do?
In a nutshell, ABECMA is a type of treatment that uses patients’ immune cells (T cells). These T cells are genetically modified with CAR to target a specific protein called B-cell maturation antigen (BCMA) in cancer cells. By doing this, ABECMA aims to enhance the body’s natural defenses.
Multiple myeloma clinical indications for ABECMA CAR-T therapy
Age groups: Adults
Medical conditions: Relapsed or refractory multiple myeloma
- Patients undergone four or more prior lines of therapy such as immunomodulatory agent,
- a proteasome inhibitor, and
- an anti-CD38 monoclonal antibody.
Dosage and administration of ABECMA CAR T therapy in multiple myeloma
Healthcare professionals administer a single dose of 300 to 460 million T cells expressing the chimeric antigen receptor (CAR) protein. This constituting the standard dosage for patients.
Safety concerns and risk management of ABECMA CAR-T therapy in multiple myeloma
CAR T therapy is administered through a controlled program called Risk Evaluation and Mitigation Strategy (REMS), effectively managing potential side effects such as
- Cytokine Release Syndrome (CRS)
- Excessive immune response
- Neurologic toxicities, and
- Hemophagocytic Lymphohistiocytosis /Macrophage Activation Syndrome (HLH/MAS)
Efficacy of ABECMA CAR T Cell therapy in multiple myeloma
So, how effective is ABECMA? Well, studies have shown promising results. The overall response rate of 72% in a group of 100 patients treated with ABECMA using 300 to 460 million CAR-positive T cells. This outcome indicates that a significant number of patients experienced positive responses.
Impact of ABECMA therapy in multiple myeloma
ABECMA is a great leap forward in medical science. It uses your body’s cells to fight against Relapsed or refractory multiple myeloma. As more research and development progress, we expect to see even better outcomes from this cutting-edge treatment.
BREYANZI CAR T therapy for Lymphoma
In medical advancements, breakthrough therapies are constantly emerging to combat challenging diseases. BREYANZI, which holds promising results for patients with Large B-cell lymphoma (LBCL).
Let’s explore the types of therapies involved, the safety measures taken, and the impressive efficacy rates witnessed.
| Name | Trade name | Manufacturer |
| lisocabtagene maraleucel | BREYANZI | Juno Therapeutics, Inc., a Bristol-Myers Squibb Company |
BREYANZI is a groundbreaking autologous cell therapy that utilizes the power of immunotherapy to combat Large B-cell lymphoma (LBCL).
Let’s explore the amazing world of modern treatments and discover how CAR T therapy can greatly change the way we treat lymphoma.
By changing a patient’s own T cells using genetic engineering, this therapy gives these cells the power to target and attack cancer cells that have a specific protein called CD19. This CD19 protein is found on the surface of a type of lymphoma cells.
Defining BREYANZI: A Precision Approach
BREYANZI is a therapy that uses changed immune cells to find and kill lymphoma cells that have certain proteins on them. The therapy puts these changed cells back into the patient’s body to locate and destroy the cancer cells.
This treatment is very personalized and effective because it uses the body’s natural defenses to fight the cancer.
Lymphoma clinical conditions for BREYANZI CAR-T therapy
Age Group: Adults
Medical Conditions:
BREYANZI is a medicine for grown-ups who have certain kinds of a condition called large B-cell lymphoma (LBCL). These include diffuse large B-cell lymphoma (DLBCL) that is not specified in any other way, high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B.
BREYANZI is suitable for adults who meet one of the following criteria:
Their disease is not responding to initial chemoimmunotherapy or has relapsed within 12 months after the first-line chemoimmunotherapy.
Their disease did not respond to the initial chemoimmunotherapy, or it relapsed after first-line chemoimmunotherapy, and they are ineligible for hematopoietic stem cell transplantation (HSCT) due to other health issues or age.
They have experienced a relapse or their disease is not responding after receiving two or more lines of systemic therapy.
It’s important to note that BREYANZI should not be used to treat patients with primary central nervous system lymphoma.
Dosage and Administration of BREYANZI CAR T therapy in lymphoma patients
The dosage of BREYANZI varies depending on the stage of the disease and the number of previous therapies.
For patients with LBCL who have undergone one line of therapy
- Recommended dosage is 90 to 110 million CAR-positive viable T cells
- Administered through intravenous infusion.
In cases of relapsed or refractory LBCL after two or more lines of therapy
- the dosage ranges from 50 to 110 million CAR-positive viable T cells
This tailored dosing approach ensures the therapy is optimized for each patient’s unique condition.
Safety concerns and risk management of BREYANZI CAR T therapy in lymphoma patients
To ensure the utmost safety, BREYANZI therapy is provided under a restricted program known as the Risk Evaluation and Mitigation Strategy (REMS).
This program focuses on managing potential side effects such as
- Cytokine Release Syndrome (CRS)
- Neurologic toxicities
By closely monitoring patients and implementing preventive measures, healthcare professionals can effectively mitigate these risks.
Efficacy of BREYANZI CAR T cell therapy in lymphoma
The efficacy of BREYANZI treatment has demonstrated remarkable outcomes for LBCL patients. Let’s take a closer look at the response rates witnessed in two specific populations:
Large B-cell lymphoma (LBCL) patients who received 90 to 110 million CAR-positive T cells:
- An overall response rate of 80% was observed among 61 patients
Relapsed or Refractory Large B-cell lymphoma (LBCL) patients who received 50 to 110 million CAR-positive T cells:
- Among 192 patients falling into this category, an impressive overall response rate of 73% was achieved.
Impact of BREYANZI therapy in lymphoma
BREYANZI, a CD19-directed genetically modified autologous T cell immunotherapy, represents a significant breakthrough in the treatment of Large B-cell lymphoma. With its targeted approach and remarkable efficacy rates, this therapy offers hope to patients who have previously faced limited options.
By leveraging the power of the patient’s own immune system, BREYANZI paves the way for a brighter future in the fight against LBCL.
CARVYKTI CAR T therapy for multiple myeloma
CARVYKTI specifically designed to combat relapsed or refractory multiple myeloma in adult patients who have undergone four or more prior lines of therapy. With its targeted approach, leveraging the power of autologous treatment, CARVYKTI is changing lives and rewriting the future of healthcare.
| Name | Trade name | Manufacturer |
| ciltacabtagene autoleucel | CARVYKTI | Janssen Biotech, Inc |
CARVYKTI represents a new frontier in medical science, B-cell maturation antigen (BCMA)-directed genetically modified autologous T cell immunotherapy. In simple terms, it harnesses the power of your immune system to tackle multiple myeloma head-on.
Multiple myeloma clinical indications for CARVYKTI CAR-T therapy
Age groups: Adults
Medical conditions: Relapsed or refractory multiple myeloma
- Patients undergone four or more prior lines of therapy such as immunomodulatory agent,
- a proteasome inhibitor, and
- an anti-CD38 monoclonal antibody.
Dosage and administration of CARVYKTI CAR T therapy in multiple myeloma patients
The dosage of CARVYKTI is precisely tailored to body weight, with a range of 0.5 to 1 × 106 CAR-positive T cells per kilogram. These genetically modified T cells act as warriors, honed to seek out and destroy cancerous cells, offering you a renewed chance at a healthier future.
Safety concerns and risk management of CARVYKTI CAR T therapy in multiple myeloma patients
Now, let’s address an important aspect: safety. Administration of CARVYKTI performed under Risk Evaluation and Mitigation Strategy (REMS). This comprehensive framework ensures to the management of potential side effects, offering you the highest level of care throughout your treatment journey.
It’s important to be aware of potential complications, such as
- Neurologic toxicities
- Neurologic toxicities, Cytokine Release Syndrome (CRS)
- Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome (HLH/MAS)
- Parkinsonism, and Guillain-Barré syndrome
- Prolonged and recurrent cytopenias with bleeding and infection
In rare cases, patients may require stem cell transplantation for hematopoietic recovery following CARVYKTI treatment.
Efficacy of CARVYKTI CAR T cell therapy in multiple myeloma
The impact of CARVYKTI on patients’ lives cannot be overstated. A study involving 97 patients treated with CARVYKTI showed an astounding overall response rate of 97.9%. This finding highlights the remarkable effectiveness of CARVYKTI, instilling hope in patients seeking a transformative and effective treatment option.
Impact of CARVYKTI therapy in multiple myeloma
CARVYKTI represents a new era in personalized therapy, showcasing the immense potential of genetic modifications in treating cancer. By harnessing the power of the body’s immune system, CARVYKTI offers a targeted and effective treatment option for patients with relapsed or refractory multiple myeloma in adult patients.
Being mindful of potential risks and complications associated with CAR T therapy is essential. However, the remarkable efficacy demonstrated by CARVYKTI underscores its potential to transform patients’ lives and pave the way for future advancements in cancer treatment.
KYMRIAH CAR T therapy for lymphoma
KYMRIAH therapy has shown remarkable success in combating h B-cell precursor acute lymphoblastic leukemia (ALL), large B-cell lymphoma, and follicular lymphoma (FL). Join us as we explore the incredible potential of KYMRIAH therapy and its impact on patients’ lives.
| Name | Trade name | Manufacturer |
| tisagenlecleucel | KYMRIAH | Novartis Pharmaceuticals Corporation |
What is KYMRIAH Therapy?
KYMRIAH therapy is an advanced form of immunotherapy that harnesses the power of a patient’s immune system to fight B-cell precursor acute lymphoblastic leukemia (ALL), large B-cell lymphoma, and follicular lymphoma (FL). It involves genetically modifying the patient’s T cells to target CD19, specifically target cancer cells.
By genetically reprogramming these cells, KYMRIAH therapy enhances the immune system’s ability to recognize and destroy cancer cells, offering a personalized and targeted approach to treatment.
Lymphoma clinical indications for KYMRIAH CAR-T therapy
Age groups: Adults
Medical conditions: Follicular lymphoma after two or more lines of therapy
Dosage and administration of KYMRIAH CAR T therapy in lymphoma patients
KYMRIAH therapy is administered intravenously and tailored to meet individual needs.
For pediatric or young adult patients with B-cell ALL, a single dose of KYMRIAH contains a specific number of CAR-positive viable T cells based on their body weight.
- Patients weighing 50 kg or less receive a range of 0.2 to 5.0 million CAR-positive viable T cells per kg body weight
- Patients weighing more than 50 kg receive 1 to 25 million CAR-positive viable T cells per kg body weight
In adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL)
- A single dose of KYMRIAH contains 6 to 60 million CAR-positive viable T cells.
Safety concerns and risk management of KYMRIAH CAR T therapy in lymphoma patients
To ensure your well-being during KYMRIAH therapy, a strict Risk Evaluation and Mitigation Strategy (REMS) is in place. This program closely monitors and manages potential side effects such as Cytokine Release Syndrome (CRS) and Neurologic toxicities, allowing healthcare professionals to provide the best possible care and ensure your safety throughout the treatment process.
The efficacy of KYMRIAH CAR T cell therapy in lymphoma
The results of KYMRIAH therapy have been nothing short of astounding. In clinical trials, KYMRIAH demonstrated remarkable efficacy across various patient populations.
In pediatric and young adult patients with relapsed or refractory B-cell ALL, 83% achieved a complete response rate, offering renewed hope and improved outcomes.
Additionally, in adult patients with relapsed or refractory DLBCL, KYMRIAH therapy yielded an overall response rate of 50%, showcasing its potential to tackle challenging cases.
Similarly, in adult patients with relapsed or refractory FL, KYMRIAH therapy achieved an impressive overall response rate of 86%.
Impact of KYMRIAH therapy in lymphoma
KYMRIAH therapy is a groundbreaking approach that empowers your immune system to fight against cancer. By harnessing the potential of genetically modified T cells, KYMRIAH offers personalized and targeted treatment options for patients battling B-cell precursor acute lymphoblastic leukemia, large B-cell lymphoma, and follicular lymphoma.
TECARTUS CAR T therapy for lymphoma and leukemia
TECARTUS an autologous, cell-based immunotherapy, offers hope to adult patients with relapsed or refractory mantle cell lymphoma (MCL) and relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
| Name | Trade name | Manufacturer |
| brexucabtagene autoleucel | TECARTUS | Kite Pharma, Inc. |
Understanding TECARTUS CAR-T Therapy
TECARTUS therapy modifies the patient’s T cells to target the CD19 protein on the cancer cell’s surface. Infusing these modified T cells into the patient’s body enhances their ability to identify and eliminate cancer cells. This approach turns TECARTUS into personalized and focused immunotherapy.
Lymphoma and leukemia clinical indications for TECARTUS CAR-T Therapy
Age group: Adults
Doctors use TECARTUS therapy for adults with relapsed or refractory mantle cell lymphoma (MCL). Additionally, it has a new indication for adult patients who have relapsed or refractory (r/r) B-cell precursor acute lymphoblastic leukemia (ALL).
Dosage and Administration of TECARTUS CAR T Therapy in lymphoma and leukemia patients
Doctors give TECARTUS to patients through an intravenous (IV) injection, adjusting the dosage based on the specific condition.
- Recommended dose is 2 million CAR-positive viable T cells per kg body weight,
- Maximum of 200 million CAR-positive viable T cells.
For those with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL), the dose is 1 million CAR-positive viable T cells per kg body weight, with a maximum of 100 million CAR-positive viable T cells.
Safety concerns and risk management of TECARTUS CAR T Therapy in lymphoma and leukemia patients
The administration of CAR T therapy, including TECARTUS, is carefully managed through a Risk Evaluation and Mitigation Strategy (REMS). The REMS strategy ensures the safety of patients by closely monitoring and managing potential side effects.
The two main concerns during TECARTUS therapy are Cytokine Release Syndrome (CRS) and Neurologic toxicities. By implementing a restricted program, healthcare professionals take proactive measures to mitigate these risks effectively.
Efficacy of TECARTUS CAR T cell therapy in lymphoma and leukemia patients
TECARTUS therapy has shown promising results in clinical trials. Researchers observed an impressive overall response rate of 87% TECARTUS therapy effectiveness in a study with 60 adult patients who had relapsed or refractory MCL. The results demonstrate the therapy’s effectiveness in combatting this challenging form of lymphoma.
Moreover, TECARTUS treatment led to an overall complete remission rate of 64.8%in a separate study with 54 patients suffering from relapsed or refractory B-cell precursor ALL. Such results offer renewed hope for patients facing this aggressive leukemia.
Impact of TECARTUS Therapy in lymphoma and leukemia patients
The impressive efficacy and careful safety management make TECARTUS a revolutionary breakthrough in cancer treatment.
YESCARTA CAR T therapy for lymphoma
When battling large B-cell lymphoma, YESCARTA therapy offers hope for patients with exhausted treatment options. YESCARTA is making waves in the medical field as a groundbreaking form of autologous, cell-based immunotherapy. Let’s dive deeper into this remarkable treatment and explore its effectiveness in combating lymphoma.
| Name | Trade name | Manufacturer |
| axicabtagene ciloleucel | YESCARTA | Kite Pharma, Inc. |
What is YESCARTA Therapy?
YESCARTA therapy is a cutting-edge form of immunotherapy that harnesses the power of the patient’s body’s immune system. In addition, YESCARTA targets and attacks CD19-positive cancer cells in large B-cell lymphoma by genetically modifying autologous T cells. This personalized approach is revolutionizing how we treat this aggressive form of cancer.
Lymphoma clinical indications for YESCARTA CAR-T therapy
Age group: Adults
TECARTUS therapy treats adult patients with large B-cell lymphoma that does not respond to first-line chemoimmunotherapy or relapses within 12 months after the first-line treatment. However, it’s not advisable to use TECARTUS therapy for treating patients with primary central nervous system lymphoma.
Dosage and Administration of YESCARTA CAR T therapy in lymphoma patients
Doctors give YESCARTA therapy through an intravenous injection, customizing the dosage for each patient’s needs.
Typically, patients receive a dose of 2 × 10^6 CAR-positive viable T cells per kilogram of body weight, up to a maximum of 2 × 10^8 CAR-positive viable T cells.
This personalized dosing ensures optimal effectiveness and safety.
Ensuring Safety and Management of YESCARTA CAR T therapy in lymphoma patients
YESCARTA CAR T cell therapy is supervised through a Risk Evaluation and Mitigation Strategy (REMS) to safeguard patient safety. Above all, REMS comprehensive program focuses on managing potential side effects such as Neurologic toxicities and Cytokine Release Syndrome (CRS). Most importantly, healthcare professionals can effectively mitigate these risks by closely monitoring patients and providing appropriate medical interventions.
Efficacy of YESCARTA CAR T cell therapy in lymphoma patients
The effectiveness of YESCARTA therapy has been demonstrated through numerous clinical trials and studies.
- In a study involving 101 patients with relapsed or refractory large B-cell lymphoma, YESCARTA exhibited an impressive objective response rate of 72%
For this reason, YESCARTA CAR T cell therapy showcases the potential to provide meaningful and life-changing outcomes for patients.
Furthermore, YESCARTA has also shown remarkable efficacy in treating relapsed or refractory follicular lymphoma.
- In a study comprising 81 patients, the therapy achieved an astounding objective response rate of 91%.
These results highlight the significant impact YESCARTA can have on patients’ lives by offering hope and the possibility of remission.
Impact of YESCARTA in lymphoma patients
With its ability to harness the body’s immune system to target and destroy cancer cells, YESCARTA represents a promising frontier in lymphoma treatment. Through personalized, autologous cell therapy, YESCARTA offers hope to those who have not responded to conventional treatments or experienced relapse.
As research and advancements continue, YESCARTA CAR T cell therapy has the potential to revolutionize cancer care and improve patient outcomes worldwide.
Ongoing CAR-T cell therapy in Clinical trials
Currently, there are 1008 clinical trials underway worldwide. For instance, the United States alone has 598 ongoing clinical trials (including all trial phases). All in all, these clinical trials aim to evaluate the effectiveness of CAR-T therapy in addressing various conditions.
(Reference: www.clinicaltrials.gov, Note: Updated as on 14 June 2023)
The conditions currently being evaluated
- B-cell precursor acute lymphoblastic leukemia (ALL)
- Multiple myeloma
- Follicular lymphoma
- Diffuse large B-cell lymphoma
- Primary mediastinal large B-cell lymphoma
- Large B-cell lymphoma transformed from follicular lymphoma
- High-grade B-cell lymphoma
- Aggressive B-cell lymphoma not otherwise specified (NOS)
- Mantle cell lymphoma
- Follicular lymphoma
(Reference: www.clinical trials.org, Note: Updated as on 14 June 2023)
Please remember that these clinical trials are conducted by dedicated professionals who are working tirelessly to find potential treatments. While the journey towards effective therapies takes time, their efforts give hope to many individuals facing these conditions.
Managing adverse and side effects
CAR T therapy can have adverse effects, and it’s essential to be aware of them. Please remember that well-prepared healthcare professionals handle these challenges and provide support throughout the patient treatment journey. Let’s explore some common CAR T therapy side effects, so you know what to expect.
The most frequently reported adverse effects include Cytokine Release Syndrome (CRS), Neurologic toxicities, and Prolonged Cytopenia with bleeding and infection. However, it is important to note that not everyone will experience all these side effects, and their severity can vary for each person.
Some of the usual side effects patients may experience during CAR T therapy include fever, tiredness, trouble breathing, shivering, confusion, difficulty talking or slurred speech, feeling sick, throwing up, diarrhea, headache, wooziness, faintness, and fast or irregular heartbeat. These symptoms might worry patients. By all means, remember that the well-prepared medical team handles and treats side effects effectively.
To ensure your safety and well-being, healthcare professionals follow a Risk Evaluation and Mitigation Strategy (REMS) plan. For this reason, healthcare professionals assess and minimize potential risks associated with CAR T therapy. Above all, it includes close monitoring of patient condition, prompt intervention if adverse effects arise, and continuous communication to address any concerns or questions.
Conclusion
Each patient medical team is experienced in handling these situations and will provide the necessary support and care every step of the way. Their expertise and dedication are there to ensure your well-being and help you achieve the best possible outcome from CAR T therapy.
It’s normal to feel anxious or overwhelmed when identifying or facing potential side effects, but it’s crucial to remember that individual patients are not alone in this journey.
Take comfort knowing you have a team of healthcare professionals fully committed to your safety and recovery. Together, you can navigate through any challenges that may arise and work towards a positive outcome. Stay strong and trust in the expertise of your medical team.
FAQ
Chimeric antigen receptor (CAR) T cell therapy is an immunotherapy that uses genetically engineered T cells to fight cancer.
T cells normally help the body to fight infection. In CAR T therapy, T cells are taken from the patient’s blood and then genetically engineered to express a receptor that can recognize and bind to cancer cells.
Once the T cells are re-infused into the patient, they can multiply and attack cancer cells.
CAR T therapy targets specific proteins on the surface of cancer cells.
The engineered T cells are programmed to recognize these proteins and then bind to them.
Once the T cells bind to the cancer cells, they release chemicals that kill the cells.
CAR-T therapy effectively treats various cancers, including leukemia, lymphoma, and myeloma.
CAR T therapy is a relatively new treatment, but it has shown great promise in the fight against cancer.
You can visit the FDA website to find a clinic that offers for lymphoma, leukemia and multiple myeloma
The FDA has approved five CAR T-cell therapies for the treatment of cancer:
ABECMA for multiple myeloma
BREYANZI for Lymphoma
CARVYKTI for multiple myeloma
KYMRIAH for lymphoma
TECARTUS for lymphoma and leukemia
YESCARTA for lymphoma
CAR T cell therapy shows significant efficacy in treating leukemia in the early phases of clinical trials.
Kymriah therapy showcased its potential with high response rates in B-cell acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), and follicular lymphoma (FL) patients.
Tecartus therapy achieved remarkable response rates in mantle cell lymphoma (MCL) and B-cell precursor ALL patients.
CAR T cell therapy shows significant efficacy in treating lymphoma in the early phases of clinical trials.
Breyanzi therapy exhibited an 80% overall response rate in Large B-cell lymphoma (LBCL) patients and 73% in Relapsed or Refractory LBCL patients.
Yescarta therapy displayed an impressive objective response rate in large B-cell and follicular lymphoma patients.
CAR T cell therapy shows significant efficacy in treating lymphoma in early phases of clinical trials
CAR T therapy can have cytokine release syndrome (CRS) and neurological toxicities side effects.
CRS is a reaction that can occur when the CAR T cells start to multiply and release large amounts of cytokines, which are proteins that regulate the immune system.
Neurological toxicities can affect the brain and nervous system.
CAR T therapy is currently only available to a limited number of patients.
Eligibility criteria vary depending on the type of cancer and the patient’s overall health.
CAR T therapy is currently available at several hospitals and clinics. Visit listed companies to find out associated hospitals and clinics for ongoing clinical trials.